


On the one hand, an increase in pressure can be achieved by an increase of the gas mass. How can the pressure in the cylinder be increased? Figure: Experimental setup for investigating the quantities influencing the pressure (ideal gas law) The pressure in the cylinder can be read on a pressure gauge. The air can also be heated with a Bunsen burner. A valve is attached to the cylinder, through which air can be filled in. The position of the piston can be adjusted by a threaded rod. The cylinder contains air as an approximately ideal gas. The setup consists of a glass cylinder and a piston. The experimental setup shown below is used to investigate the variables influencing the pressure. We will consider the gas as an ideal gas. The influencing parameters will be explained in the following.
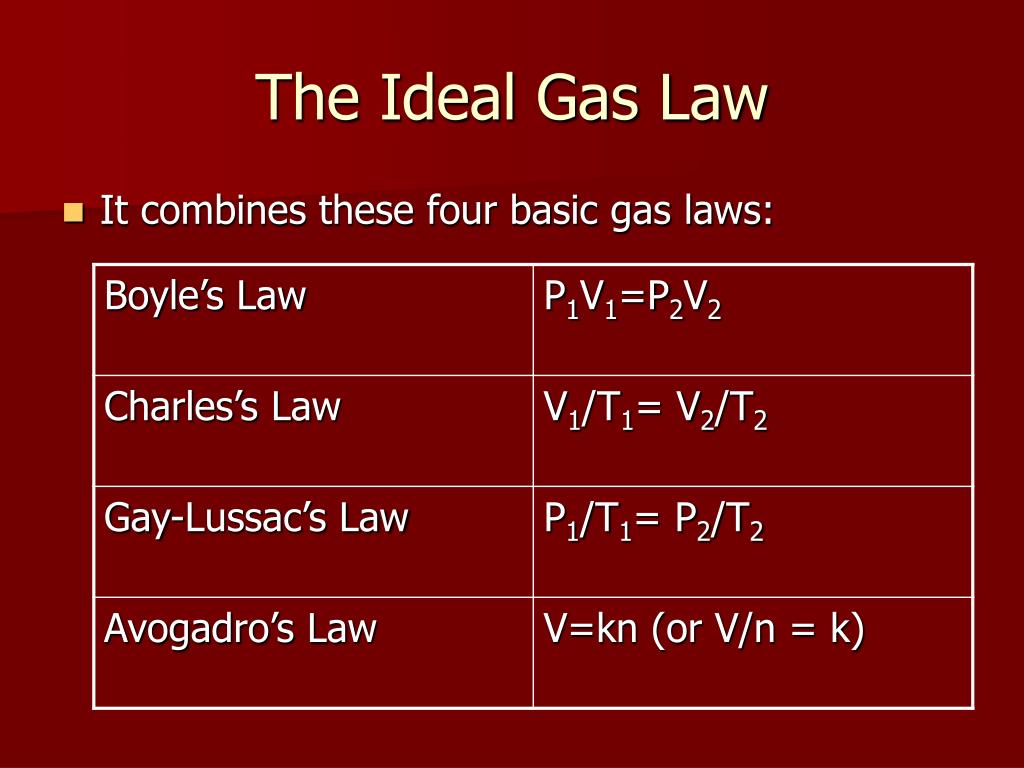
Therefore, the question arises which thermodynamic parameters have any influence on the pressure in a cylinder and which laws are the basis for this.
It is therefore of interest that heat engines generate the highest possible pressure. The higher the gas pressure, the greater the work done by the gas. Internal combustion engines (generally referred to as heat engines) also work according to the above-mentioned principle. Due to the high pressure, the gas is therefore able to do work. If, on the other hand, the piston is released, the gas expands and pushes the piston out of the cylinder with a certain force. To keep the piston in position, a counterforce must be exerted. If the pressure inside the cylinder is now increased, this becomes noticeable by a force acting on the piston ( collisions of gas molecules with the piston surface). The figure below shows a gas-filled cylinder closed with a piston. 5.3 Dependence on the number of particles.5.2 Dependence on the amount of substance.5 Alternative formulations of the ideal gas law.4 Specific gas constants of selected gases.1 Parameters influencing the gas pressure.


 0 kommentar(er)
0 kommentar(er)
